New Insights on the Mechanism of Electrocatalytic Reduction of Oxygen
(September 2019)
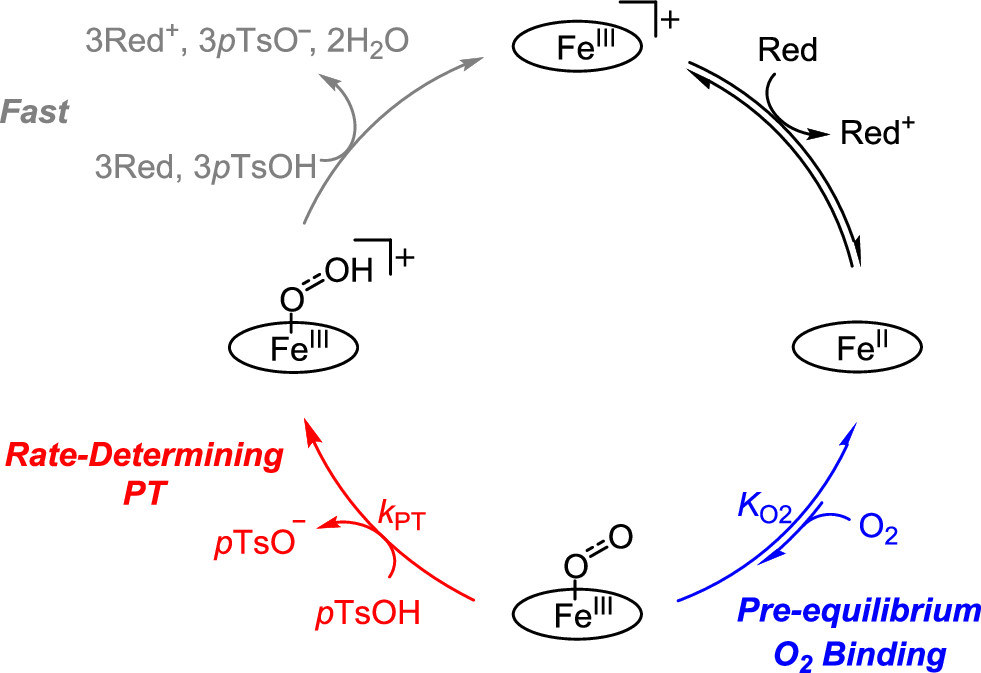
Catalytic reduction of dioxygen to water is crucially important in fuel cells, where it is coupled with oxidation of a fuel, such as dihydrogen. Recent research from the CME reveals details of the mechanism for a molecular iron electrocatalyst. The results, published in the May 2019 issue of the Journal of the American Chemical Society, combined direct observation and quantification of the pre-equilibria and rate-determining steps of three catalytic intermediates at Yale University with computational studies conducted at PNNL. The research team discovered that the rate-determining step is a proton transfer step from an acid to a dioxygen bound to iron. These results may help better understand the proton transfer dynamics in electrocatalytic reactions.