Tackling Our Energy Challenges in a New Era of Science
Latest Announcements

Morris Bullock Co-Chairs Hydrogen Roundtable
Discussion helps identify opportunities in hydrogen research
(November 2021)
Laboratory Fellow Morris Bullock, a chemist at Pacific Northwest National Laboratory, co-chaired a roundtable discussion on carbon-neutral hydrogen convened by the Department of Energy's Office of Basic Energy Sciences. Scientists and engineers from academia, industry, and national laboratories participated in the roundtable, "Foundational Science for Carbon-Neutral Hydrogen Technologies."

Morris Bullock to Be Honored as First National Laboratory Scientist to Receive American Chemical Society Award in Organometallic Chemistry
Bullock to be honored with 2022 ACS Award after more than 35 years of research
(September 2021)
The American Chemical Society (ACS) will honor Morris Bullock with the coveted 2022 ACS Award in Organometallic Chemistry as the first national laboratory employee to receive the award. Bullock is a Laboratory Fellow and chemist in the Physical Sciences Division at Pacific Northwest National Laboratory (PNNL), the director of the Center for Molecular Electrocatalysis, an Energy Frontier Research Center led by PNNL, and associate director of PNNL's Institute for Integrated Catalysis.

CME's Brandi Cossairt Elected to the Washington State Academy of Sciences
(August 2021)
Brandi Cossairt, who is a PI in the Center for Molecular Electrocatalysis (CME) and a Professor of Chemistry at the University of Washington, was elected to the Washington State Academy of Sciences (WSAS).

Karthish Manthiram Receives Two Early Career Awards
(June 2021)
Karthish Manthiram, the Theodore T. Miller Career Development Chair and an assistant professor in chemical engineering at the Massachusetts Institute of Technology (MIT), has been named both a 2021 Camille Dreyfus Teacher-Scholar and a 2021 Sloan Research Fellow.
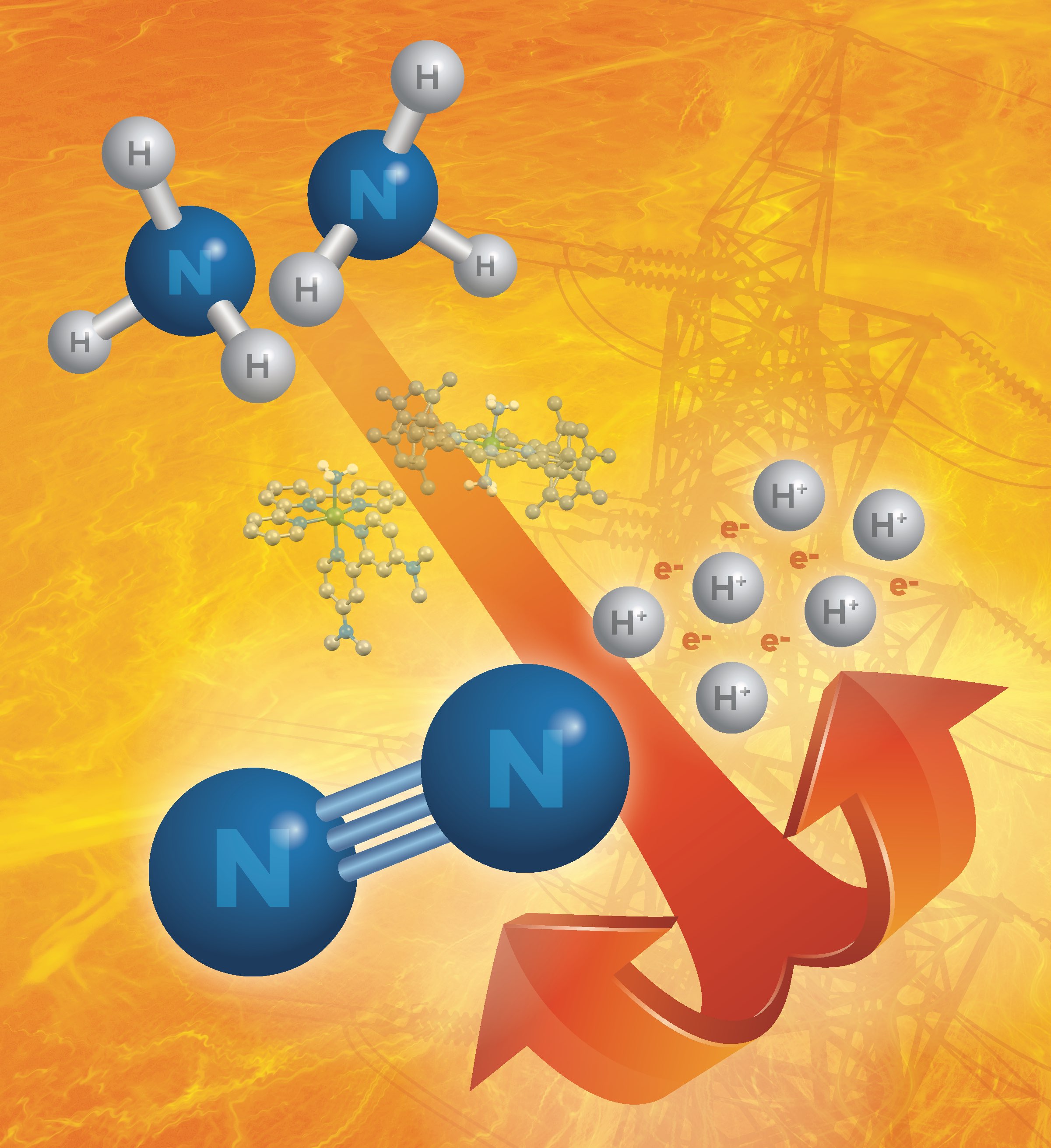
Perspective on Oxidation of Ammonia
(November 2020)
Ammonia, NH3, is an essential chemical, produced globally from the Haber process at a massive scale (165 million tons annually). A familiar component of strong household cleaners, ammonia is an energetically dense molecule that primarily turns into the fertilizers that feed the world. However, its potential for other applications requires knowledge on how to release the energy stored in the N-H bonds. A new article in the Journal of the American Chemical Society from CME researchers provides a perspective on the use of molecular catalysts to do just that by oxidizing ammonia.

CME's Hammes-Schiffer Receives Royal Society of Chemistry, American Chemical Society Awards
(October 2020)
Sharon Hammes-Schiffer, deputy director of the Center for Molecular Electrocatalysis (CME) and the John Gamble Kirkwood Professor of Chemistry at Yale University, has received awards from both the Royal Society of Chemistry and the American Chemical Society (ACS).

CME's Mayer Elected to American Academy of Arts and Sciences
(May 2020)
James Mayer, Charlotte Fitch Roberts Professor of Chemistry at Yale University and research leader at PNNL's Center for Molecular Electrocatalysis (CME), has been elected to the American Academy of Arts and Sciences.

Professor Shannon Stahl wins the 2020 American Chemical Society Catalysis Lectureship
(March 2020)
Stahl is the Steenbock Professor of Chemical Sciences at the University of Wisconsin-Madison, and he leads the Molecular Mediators thrust in the Center for Molecular Electrocatalysis. The ACS Division of Catalysis Science and Technology and the journal ACS Catalysis jointly award the lectureship annually, honoring outstanding research in catalysis.

DOE Awards Presidential Early Career Award to CME Researcher Yogesh Surendranath
(September 2019)
Yogi Surendranath was honored with a Presidential Early Career Award for Scientists and Engineers in a ceremony in Washington D.C. in July 2019.
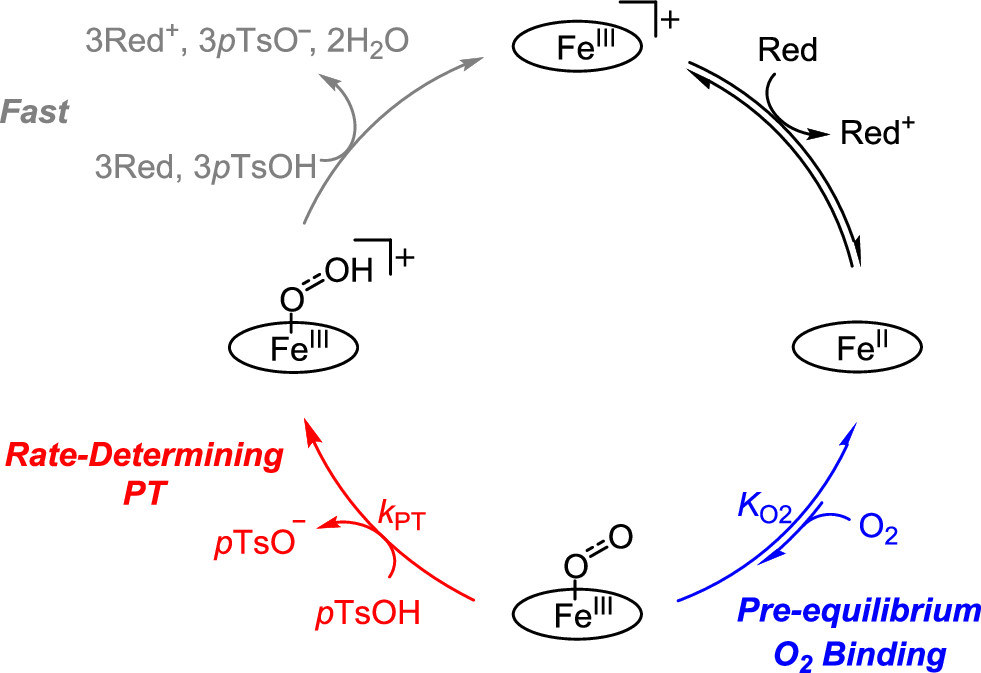
New Insights on the Mechanism of Electrocatalytic Reduction of Oxygen
(September 2019)
Catalytic reduction of dioxygen to water is crucially important in fuel cells, where it is coupled with oxidation of a fuel, such as dihydrogen. Recent research from the CME reveals details of the mechanism for a molecular iron electrocatalyst.

CME Researchers Honored with Team Science Award
(September 2019)
CME investigators Daniel Martin (Yale) and Samantha Johnson (PNNL) received a team science award at the 2019 EFRC Principal Investigators' Meeting in Washington, D.C. in July.

Mechanism of Iron-based Hydrogen Bond Cleavage Revealed
A first look at hydrogen cleavage by a new route
(February 2019)
Scientists at PNNL's Center for Molecular Electrocatalysis (CME) are working to understand the fundamental reactivity of H2 that could contribute to making hydrogen a more widely used fuel source.
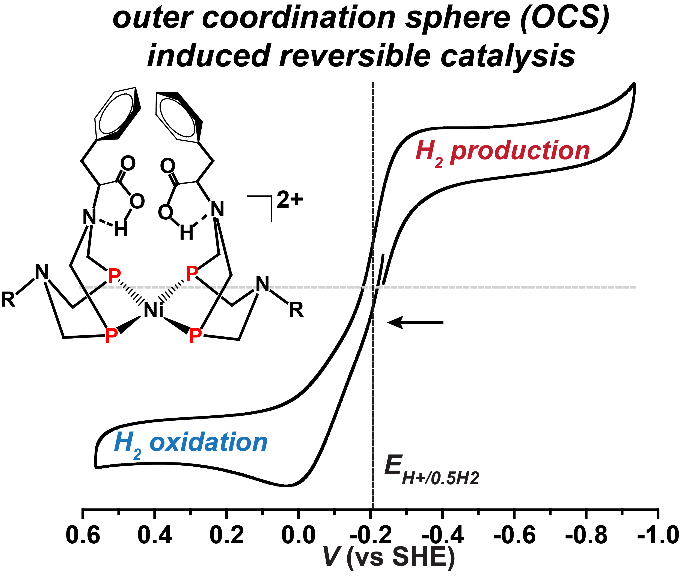
Scientists Design Fast, Reversible Bio-inspired Catalysts
New synthetic catalysts mimic desirable enzyme behavior
(February 2019)
Using a bio-inspired design that mimics nature's catalysts-enzymes-researchers at PNNL have designed a rarely seen reversible synthetic catalyst.

Max Planck Institute Names CME Researcher, Jim Mayer, 2019 Frontiers Award Recipient
(February 2019)
Jim Mayer, CME’s thrust lead for heterogeneous interfaces, was recently honored with the 2019 Frontiers Award from the Max Planck Institute. He traveled to Germany to accept the award and give four lectures at the research center.

Samantha Johnson Receives Award for Poster
(January 2019)
Samantha I. Johnson, a post-doctoral researcher working in the Center for Molecular Electrocatalysis, Catalysis Science Group (PCSD), recently won an award for her poster, "Using Computational Methods to Design Ammonia (NH3) Oxidation Catalysts for Use in Fuel Systems," from the Clean Energy Education and Empowerment (C3E) Initiative at their 2018 symposium, held in December.
Sharon Hammes-Schiffer Elected to the National Academy of Sciences
(May 2013)

Congratulations to Prof. Sharon Hammes-Schiffer, Center for Molecular Electrocatalysis, on being selected as a member of the National Academy of Sciences. A world leader in theoretical and computational chemistry, Hammes-Schiffer studies proton-coupled electron transfer reactions at the Energy Frontier Research Center, funded by DOE's Office of Basic Energy Sciences. She is the Swanlund Professor of Chemistry at the University of Illinois at Urbana-Champaign.
Established 150 years ago by President Abraham Lincoln, the National Academy of Sciences is an official adviser to our nation's government, upon request, in any matter of science or engineering. This prestigious organization furthers science through the election of its members and through original research in the Proceedings of the National Academy of Sciences.
Would You Hire This Catalyst?
(May 2013)

Given two catalysts for the job of turning intermittent wind or solar energy into chemical fuels, scientists chose the material that gets the job done quickly and uses the least energy. A catalyst that quickly produces fuel but uses far more energy than it stores won't get the job. Scientists could measure the overpotential in water but not in other liquids, until Dr. Morris Bullock and Dr. John Roberts devised a quick, elegant technique. This work was done at the Center for Molecular Electrocatalysis, an Energy Frontier Research Center, funded by DOE's Basic Energy Sciences.
Controlling Proton Source Speeds Catalyst in Turning Electricity into Fuels
(April 2013)

Scientists at the Center for Molecular Electrocatalysis demonstrated that matching the proton source's pKa to that of a nickel-based catalyst speeds the conversion of electricity to hydrogen bonds dramatically. Turning electricity into chemical bonds and vice versa is necessary to capture intermittent renewable energy as use-any-time fuel. The Center is an Energy Frontier Research Center, funded by DOE's Office of Basic Energy Sciences, and is led by Pacific Northwest National Laboratory.
Transformations Presents Catalysis and Sustainable Energy
(March 2013)

The latest issue of Transformations shows the role of catalysts in making wind, solar and other sustainable energy sources a major part of the nation's energy landscape. Dr. Dan DuBois, Deputy Director of the Center for Molecular Electrocatalysis, shares the three principles involved in creating electrocatalysts, which drive the interconversion of electricity to energy stored in chemical bonds. Learn about this research and much more at the American Chemical Society symposium being held in his honor. Applied and fundamental scientists talk about the power of theory or computational chemistry to break chemistry bottlenecks and settle basic energy questions. Don't miss the latest video – featuring the Center's Dr. Monte Helm and Dr. Morris Bullock.
Chemical Society Symposium to Honor Catalysis Research of Dan DuBois
(March 2013)

Given his scientific successes and caring personality, the opportunities to speak at the 1.5-day symposium honoring the career of Dr. Dan DuBois, Pacific Northwest National Laboratory, filled quickly. The event honors DuBois American Chemical Society's Award in Inorganic Chemistry. Dr. Aaron Appel and Dr. Monte Helm at Pacific Northwest National Laboratory, along with Dr. Jenny Yang at the Joint Center for Artificial Photosynthesis, organized the symposium.
Synthetic Molecule First Electricity-Making Catalyst to Use Iron to Split Hydrogen Gas
(February 2013)

Scientists at Center for Molecular Electrocatalysis based at Pacific Northwest National Laboratory developed a fast and efficient iron-based catalyst that splits hydrogen gas to make electricity -- necessary to make fuel cells more economical.
Adding Natural Elements to Synthetic Catalysts Speeds Hydrogen Production
(February 2013)

By grafting features analogous to those in Mother Nature's catalysts onto a synthetic catalyst, scientists created a hydrogen production catalyst that is 40% faster than the unmodified catalyst. This study provides foundational information that could, one day, help design and synthesize the catalysts for hydrogen production for fuels, long-lasting electric car batteries, and energy storage from solar and wind farms.
Proton Delivery and Removal Can Speed or Distract Common Catalyst
(February 2013)

Proton delivery and removal determines if a well-studied catalyst takes its highly productive form or twists into a less useful structure, according to scientists at the Center for Molecular Electrocatalysis, an Energy Frontier Research Center based at Pacific Northwest National Laboratory. The catalyst takes two protons and forms molecular hydrogen, or it can split the hydrogen. The team showed that the most productive isomer, endo/endo, has the key nitrogen-hydrogen bonds pushed close to the nickel center. If the catalyst is in the endo/endo form, the reaction occurs in a fraction of a second. If the catalyst is stuck in another form, the reaction takes days to complete.
A Pathway for Protons
(January 2013)

Moving four relatively large protons to where they are needed is easier if you build a path, as is being done by scientists at the Center for Molecular Electrocatalysis. The research team has built two iron-based compounds that help protons move from the exterior to where they are needed. Once delivered, the protons bond with molecular oxygen and create water. In previous compounds, the protons often don't arrive in time or go to the wrong place, which leads to forming the unwanted byproduct hydrogen peroxide. The new compounds direct the protons in ways that help separate the two oxygen atoms in O2, and thereby drive the reaction to completion.

